W.L. Pohl:
Economic Geology, Principles and Practice: Metals, Minerals, Coal and Hydrocarbons
— an Introduction to Formation and Sustainable Exploitation of Mineral Deposits
(book published May 2011)
Sample Chapter:
Economic Geology of Niobium and Tantalum (Update 1st July 2016)
| Density (g/cm3) | |||
| Niobite (niobium-rich member of the columbite series) | (Fe,Mn)(Nb>Ta)2O6 | 31-79% Nb2O5 | min. 5.2 |
| Tantalite (tantalum-rich member of the columbite series) | (Fe,Mn)(Ta>Nb)2O6 | 52 to 86% Ta2O5 | max. 7.9 |
| Pyrochlore | (Na,Ca)2(Nb,Ti,Ta)2O6(O,OH,F) +3-6% REE-oxides | 56-73% (Nb,Ta)2O5 | 4.2-4.5 |
| Microlite (tantalum-rich end member of the pyrochlore series) | (Na,Ca)2Ta2O6(O,OH,F) | max. 80% Ta2O5 | 6.3 |
| Wodginite | MnSnTa2O6 | max. 70% Ta2O5 | 7.3 |
Note that pure niobite and tantalite are unknown in nature; they are theoretical end members of a solid solution series called columbite group minerals (CGM), or short, columbites. Typically, CGM form the recoverable part of a large number of minor Ta�Nb oxides (TNO) in the ore (Melcher et al. 2015). Tantalite is defined by an Nb/Ta atomic ratio <1. Before discovery of pyrochlore in carbonatites of Brazil and Canada, columbite was the main source for both tantalum and niobium.
The chemical composition of columbites is highly variable (Melcher et al. 2015, Melcher et al. 2016). Often, the �columbite quadrilateral� is used to depict changes in the Fe/Mn and Nb/Ta ratios (e.g. Beurlen et al. 2008). Preponderance of either Fe or Mn is noted by a suffix, e.g. �tantalite-Mn� (Burke 2008). With increasing fractionation of pegmatites, columbites are enriched in tantalum and manganese. Columbites contain traces of numerous elements, including As, Bi, Sn, W, Ti, Hf, Sc, REE, V, Pb, Zn, Zr, Th and U (Lehmann et al. 2014). In concentrates, the latter may enforce radiation protection measures. Inclusions of columbite and other Ta-minerals in cassiterite of pegmatite deposits are frequent. As columbite is initially free of lead and because of its resistance to later alterations, the mineral is useful for U-Pb dating of granites and pegmatites (Romer et al. 2007). Scandium in columbite and related minerals may reach >6 wt. % (Kempe & Wolf 2005).
Scandium (density 3.0 g/cm3, melting point 1539�C) is a typical dispersed element. Its Clarke value (25 ppm) is higher than that of tungsten or lead (1.2 and 13 ppm, respectively) but concentrations and minerals of this element are extremely rare (e.g. thortveitite, a silicate of scandium and yttrium with the formula (Sc,Y)2Si2O7, traces of which occur in granitic pegmatites). Like Y, scandium is one of the �pseudolanthanides� (cf. chapter �Rare Earth Elements�). In igneous rocks, trace contents of Sc3+ are positively correlated with and substitute Fe2+ in clinopyroxene, amphibole, biotite and ilmenite. High-silica magmatic rocks have low concentrations (3-5 ppm). Scandium may be enriched to an economically recoverable tenor of ca. 300 (100-500) ppm in laterite, or to by-product grades in bauxite, lateritic nickel ore, phosphorites, titanium placers, columbite concentrate, wolframite, cassiterite, uraninite and in zircon (Wood & Samson 2006, Kempe & Wolf 2005, Wiesheu et al. 1997). Cut-off grade is typically at 100 ppm. Among other applications, scandium is useful as a grain refiner in high-strength aluminium alloys (e.g. in sports equipment), and in solid oxide fuel cells (SOFCs), which convert stored chemical energy (such as hydrogen or hydrocarbons) to useable electrical energy. Annual world production sourced from China, Kazakhstan, Russia and Ukraine is estimated at 10-15 tonnes (USGS 2016).
Pyrochlore is chemically quite variable; for example, calcium can be substituted by Ba, LREE and U. Therefore, pyrochlore tends to be radioactive and metamict, i.e. its crystal structure is damaged by radiation. Often, its paragenesis includes minerals of Ti, U, Th and REE. Bariopyrochlore is the main ore mineral at Arax� (see below). Formerly in Russia, niobium was produced from loparite concentrates, derived from nepheline syenites of the Kola Peninsula. Loparite is a perowskite (CaTiO3) containing Nb and Ce. Compared with pyrochlore, however, loparite is economically not competitive. Exploitable niobium ores haveminimal grades of about 0.3%, but ore at Arax� contains 2.5% Nb2O5. Tantalum ore has characteristically grades of about 0.03% Ta2O5, generally as a co-product of cassiterite and other minerals. Note that because of facile overgrinding, metallurgical recovery of columbite by crushing and milling hard-rock ore is often as low as 50%.
Tantalum is silvery grey, heavy and very hard but malleable and ductile. It has a high melting point exceeded only by tungsten and rhenium. Tantalum alloys with other elements have great strength, good ductility and high melting points. Niobium is mainly noted for its enhancement of strength and toughness in stainless steel (Tantalum-Niobium International Study Center).
| Density | Melting Point | Boiling Point | |
| Tantalum | 16.7 | 2996�C | 5429 �C |
| Niobium | 8.57 | 2477�C | 4927�C |
Most niobium is consumed for production of micro-alloyed steel (with ~0.03% Nb) for manufacturing high-pressure pipeline tubes, offshore petroleum drilling and exploitation platforms, and automobiles. Nickel-free stainless steel contains 0.5, and superalloys ca. 5% niobium (e.g. for jet engines). Niobium-titanium (-zirconium) alloys are used in supraconducting magnets. Some niobium, but 65% of tantalum supply is destined for the electronic sector. Most electronic-grade tantalum metal is used as a capacitor core, together with some Ta-pentoxide as a dielectric barrier. Tantalum is essential for portable electronic devices such as mobile phones, laptop computers, digital cameras and navigation systems in cars and aeroplanes. The remainder of tantalum production serves manufacturing cutting tools (tantalum carbide), high-temperature alloys (e.g. turbines of gas-fired power stations), and of extremely corrosion-resistant equipment for the chemical industry.
There are also medical applications (e.g. implants), based on tantalum�s total inertness to body fluids (tantalum, similar to niobium, only dissolves in hydrofluoric or fuming sulphuric acid). Tantalum has no role in biochemical processes nor is it hazardous, with the possible exception of occupational exposure. Environmental problems of Ta-Nb mining concern foremost physical disturbance of land; toxic hazards are limited (Lehmann et al. 2014). Among them are somewhat elevated trace contents of arsenic (104 ppm in Greenbushes� albite zone which hosts the Ta ore: Partington et al. 1995) and uranium (16.3 ppm). Spring water seeping from tailings should be controlled. Landscaping and restoring ecosystems (cf. Chapter 5.4) is the main task.
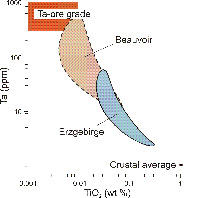
- Figure 1.18: Ta/TiO2 variation of granites from the northern French Massiv Central and the Saxo-Bohemian Erzgebirge (modified from Lehmann 1990). Tantalum (an incompatible element) is enriched to varying tenors that depend on the degree of fractionation of individual intrusions. Some samples from Beauvoir reach exploitable grades. Concurrently, contents of compatible titanium decrease. Derivation of tantalum from geochemically ordinary crust via partial melting and mineral fractionation appears possible.
The geochemical behaviour of niobium and tantalum in petrogenetic processes is nearly identical. Tantalum was discovered in 1802 by A. G. Ekeberg in Stockholm and named after the Lydian king Tantalos, father of Niobe (see wikipedia.org/wiki/Tantalos) because of its resistance to the most elaborate chemical digestion methods. Only about 50 years later, tantalum and niobium could be clearly discerned. Both are incompatible and lithophile high-field-strength elements (HFSE). Remember that HFSE form relatively small cations (Ta(V) = 88 compared to LIL Cs(I) = 188 and tiny lithophile Be(II) = 41 pm; 1 pm (picometre) = 1 x 10-12 metre: WebElements: the periodic table on the web ), which develop intense electrostatic fields that strongly reduce their potential to substitute for more common elements in rock-forming minerals. This is further inhibited by high activity of complexing volatile compounds (B, C, Cl, F, P) that cause the HFS to collect in late liquid phases.
Mantle melts (alkali-carbonatitic, but also basalts) extract both elements into the crust, compared with primitive mantle, although Nb is preferrred over Ta (Chakhmouradian 2006). Preferential titanite over rutile melting in subducted oceanic crust generates melts with very high Nb/Ta (John et al. 2011). With a crustal abundance of 12-20 ppm, niobium is more common than tantalum (1-2 ppm; GERM Earth Reservoir Database ). Both elements are enriched in highly differentiated granites, in alkali granites and syenites, in carbonatites and in rare-metal granites and pegmatites. Niobium outweighs tantalum in carbonatites and syenites, whereas in granites and pegmatites tantalum reaches higher concentration (cf. �tin granites�; Figure 1.18). In peraluminous granites (i.e., with an Al2O3/(CaO + Na2O + K2O) ratio (A/CNK) >1), Nb/Ta ratios vary from <2 to 16 (average continental crust = 12). Generally, magmatic differentiation and fractional crystallisation decrease Nb/Ta. Nb is slightly more mobile than Ta, suggesting that magmatic-hydrothermal processes account for the decrease of the Nb/Ta ratio to <5% in peraluminous granites that may be related to Ta, Cs, Nb, Be, Sn and W mineralisation (Ballouard et al. 2016). This hypothesis is, however, strongly contradicted by Stepanov et al. (2016) who provide arguments for a purely magmatic enrichment of Ta. The enrichment of Ta by fractional crystallization of granites and pegmatites is mainly controlled by mineral-melt partition coefficients (Linnen & Cuney 2005).
As a result of fractionation, columbite saturation in H2O, B, F, P, � Li enriched granitic melt at 600oC is reached at 2000-4000 ppm Ta. Peralkaline melt inclusions in spodumene from Capoeira-2 pegmatite, Borborema, Brazil, display concentrations of 10,000 ppm Ta2O5; these melts consist of ~30% NaHCO3 and 13% Li2CO3, apart from silica, alumina, potassium and water (Thomas et al. 2011). Ore of this grade, however, is rare; common tenors are 300-1000 ppm only. In the London (2008) model of pegmatite formation, this contradiction is resolved by invoking drastic undercooling to temperatures well below the equilibrium solidus. Oscillating concentrations at the solid/liquid boundary layer in the undercooled melt induce precipitation of Ta oxides, often in banded albitite.
Thomas et al. (2012) refute London�s constitutional zone refining and undercooling model and propose that the rare element minerals crystallise from highly fractionated liquid that is flux-rich, hydrous, sodic, enriched in alkali carbonates and bicarbonates, lithium and other rare elements. Note, however, that columbites crystallize from melts and not from hydrothermal fluids (Lichtervelde et al. 2007) although immiscible fluids are part of the liquids preserved in Borborema and numerous other pegmatites (Thomas et al. 2012, 2011). In sub-solidus hydrothermal systems, niobium and tantalum are extremely immobile (Linnen 1998) except in concentrated high-T, reduced fluoride solutions (Zaraisky et al. 2010) that may produce secondary mica and greisen rocks. Industrial recovery methods underline the inertness of tantalum and niobium: ore is fused with alkalis and dissolved in concentrated fluoric acid followed by liquid-liquid separation of the resulting complex Ta-Nb fluoride salt solutions. Fluorine alone has little effect on Ta and Nb solubility in granitic melt (Fiege et al. 2011). Columbite is very resistant to exogenic chemical alteration but is mechanically reduced to fines by alluvial transport. Accordingly, only residual and proximal eluvial/alluvial placers are preserved.
Niobium and Tantalum Deposit Types
Although the twin elements are never totally separated, Nb-ore forms mainly from melts extracted from the subcontinental mantle, typically during extensional tectonics, whereas Ta is �distilled� by partial melting from the continental crust, during collisional or late-orogenetic anatexis. Most of the world's Ta is produced from peraluminous pegmatites. Amalgamation of supercontinents appears to control the timing of important Ta deposits (as shown by U/Pb dating of CGM: Melcher et al. 2015, Melcher et al. 2016).
- Nb>Ta: Lateritic regolith (residual supergene enrichment blankets) above carbonatite intrusions (Arax�, Brazil, potentially Mt. Weld, Australia)
- Nb>Ta: Hard rock carbonatite or nepheline syenite with pyrochlore (Niobec mine Quebec, Canada); even small tantalum tenors improve the viability of such deposits, whereas elevated concentrations of radioactive minerals are detrimental;
- Ta>Nb: Rare-element granites ("tantalum granite") with tantalum-rich magmatic columbite and cassiterite (Yichun, South China: Yin et al. 1995; Figure 1.15; potential future mines include Ghurayyah in Saudi Arabia, Abu Dabbab in Egypt)
- Ta>Nb: Rare-element pegmatites of the Li-Cs-Ta type with tantalum-rich magmatic columbite and magmatic to hydrothermal cassiterite (examples are today's largest tantalum mine Wodgina and, until closure in 2006, Greenbushes in Australia: compare chapter �Lithium� and Figure 2.41)
- Ta>Nb: Eluvial, alluvial and coastal tin placers (Malaysia, Nigeria, Central Africa).
Alkali complexes with carbonatites and nepheline syenites occur along continental rifts or in zones of extensional crustal thinning. However, the primary hard and low-grade rocks are rarely mined. More than 90% of the mines extract residual supergene enrichment blankets. At Arax� in Minas Gerais, Brazil, unweathered carbonatite only contains about 1.5% Nb2O5 compared to regolith ore with 2.5% Nb2O5. The ca. 90 Ma old Arax� (or Barreiro) complex (Gomes et al. 1990) has a diameter of ~5 km and is marked by positive radioactive and magnetic anomalies, highest at the centre where unweathered dolomitic carbonatite ore consists of magnetite with exsolved ilmenite, phlogopite, pyrochlore and apatite. Phoscorite with P2O5 contents up to 17% and phlogopite glimmerite is abundant. Weathering transformed primary pyrochlore into bariopyrochlore (and enriched REE and apatite) to a depth of 240 m. Reserves and resources of supergene niobium ore amount to ~450 Mt. Pyrochlore is concentrated by flotation. Tantalum contents improve the viability of such deposits, whereas elevated concentrations of radioactive elements are detrimental. Because of commonly high phophorous (± magnetite and phlogopite) contents in the protore (the unaltered carbonatites) these supergene ores are phosphate-rich and rather constitute phosphate � REE deposits like Mt. Weld with estimated resources of 273 Mt at 0.9% Nb2O5 containing 145 Mt of Ta2O5 ore at 0.034% (cf. chapter �Rare Earth Elements�, Figure 2.40). Recovery of Nb and Ta from the supergene ore at Mt. Weld is expected to comprise gravity and magnetic separation, flotation, caustic and acid leaching (Aral & Bruckard 2008). Also carbonatite-related is the origin of the giant REE-Nb-Fe deposit Bayan Obo in Northern China but details remain disputed (Smith et al. 2015; Fan et al. 2004).
Tantalum-mineralised granites and pegmatites are typically derived from highly differentiated, hydrous, residual melt batches of cooling felsic magma bodies (cf. Chapter 2 �Tin�). Similar to lithium, tantalum is enriched in portions of <0.05% of the original volume (Evensen & London 2002). The resulting rocks are marked by magmatic albite and often include portions of nearly monomineralic albitite (Figure 2.41). Post-solidification metasomatic-hydrothermal albitization was previously invoked as a genetic alternative (e.g. Schwartz 1992). In a scientific deep continental drilling program, the Late Palaeozoic lithium-tantalum-tin granite of Beauvoir in the French Massif Central was intensively investigated. Results showed that only magmatic processes including fractionation and possibly, an immiscible brine-melt phase, contributed to metal enrichment (Figure 1.18); Hydrothermal metasomatism could be excluded (Linnen & Cuney 2005, Raimbault et al. 1995). Note, however, that Beauvoir is a kaolin mine with minor by-production of cassiterite (800 g/t Sn) and columbite (190 g/t Ta plus 120 g/t Nb).
The Abu Dabbab area in the Eastern Desert between River Nile and the Red Sea in Egypt is part of the Arabian-Nubian Shield (ANS), which accreted from island-arc terranes (Johnson et al. 2011) as the northern part of the East African Orogen (Fritz et al. 2013), during final amalgamation of the Late Neoproterozoic-Cambrian supercontinent Gondwana From about 680 to 550 Ma, the new continental crust of the ANS was flooded by plutons of calc-alkaline and later, during extension, of alkaline affinity. Highly evolved members of the suite are either mineralized or associated with tantalum, tin and tungsten pegmatites, and quartz vein deposits (K�ster 2009).
Abu Dabbab is a small conical mountain (Figure/Plate 2.18) built of the apical hydrothermally altered part of a granite cupola (�apogranite�). The rock is fine-grained peraluminous leucogranite of crustal origin (K�ster 2009) consisting mainly of albite, some microcline, quartz and muscovite. Ore minerals are disseminated in the uppermost 130 m of the granite beneath the roof. The paragenesis comprises Ta-rich columbite, cassiterite, pyrochlore, monazite, rutile, zircon, magnetite, galena and sphalerite. Total resources are estimated as comprising 44 Mt of ore at 250 ppm Ta2O5, 116 ppm Nb2O5 and 0.09% Sn. A bankable feasibility study proposed to produce mainly Ta2O5, cassiterite and ceramic-grade feldspar. Similar are the nearby measured resources at Nuweibi in Egypt (Helba et al. 1997). U�Pb age estimates for tantalite of these granites suggest a Pan-African age of ~550 Ma (Melcher et al. 2015). Ghurayyah in Saudi Arabia, a mineralized peralkaline granite fractionated from mantle melts (K�ster 2009) is thought to be the world�s largest potential Ta-Nb�Zr�REE deposit (385 Mt of ore containing 94,000 t Ta2O5).
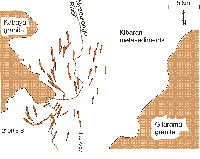
- Fig. 2.42 � Sketch map of the Kibaran (ca. 950 Ma) pegmatite swarm (red) in the Gatumba tin-tantalum mining district, Rwanda. Near Nyabarongo River, Kabaya granite attains the character of an apical tin granite. From 1928 to 1985, the district produced about 18,000 t of cassiterite and 2,000 t of columbite concentrate, from regolith ore of an average combined grade of 0.5 kg/m�
Tantalum pegmatites are not rare, with deposits known in many countries (Canada, Brazil, Ethiopia, Australia: Figure 1.20, 2.41). At present, Volta Grande (Mibra) mine in Brazil (Qu�m�neur & Lagache 1999) may be the largest pegmatite operation, producing Ta, Nb, Sn and ceramic feldspar from ore grading 375 ppm Ta2O5. The Kenticha deposit in Ethiopia is a highly fractionated subhorizontal Li-Cs-Ta pegmatite sheet with a thickness approaching 100 m that is related to Late Neoproterozoic I-type granites. Exploitable tantalum grades occur in its upper zone characterized by spodumene and high concentrations of Rb, Cs and Ga (K�ster et al. 2009).
Numerous, relatively small pegmatitic deposits are common in Rwanda, Burundi and Eastern Congo. Swarms of pegmatites form districts (e.g. Gatumba in Rwanda: Figure 2.42; Lehmann et al. 2014; Dewaele et al. 2011). The giant subhorizontal pegmatite sheet at Manono in the DR Congo (Dewaele et al. 2015) resembles the Kenticha and Bernic Lake (Canada) pegmatites. Tin-tantalum pegmatites in the Central Africa region are related to Early Neoproterozoic S-type �tin� granites of the Kibara belt (Pohl et al. 2013; Figure 1.16), which intruded during the final welding of supercontinent Rodinia (~ 986 Ma; �pan-Rodinian orogenic events�: Li et al. 2008). Varlamoff (1972) discerned lithium-rich internally zoned pegmatites with by-product beryl, tin and tantalum and unzoned albite-rich tin-tantalum pegmatites. The latter contain the ore minerals columbo-tantalite (�coltan�) and cassiterite; characteristic gangue comprises elbaite, spodumene, some lepidolite, beryl and muscovite (e.g. at Gatumba). Hulsbosch et al. (2014) suggest that at Gatumba, starting from parental granite (with K 3.1 wt%, Rb 222 ppm and Cs 11 ppm), pegmatitic K-feldspar (Rb 350�6000 ppm, Cs 2�160 ppm) and muscovite (Rb 670�7000 ppm, Cs 10�150 ppm) provide a vector towards highly differentiated mineralised Sn-Ta pegmatites. The most primitive biotite pegmatites form from 0% to 69% crystallisation, two-mica pegmatites from 69% to 92%, and muscovite pegmatites from 92% crystallisation onwards. A Rayleigh fractional crystallisation model implies that rare-element pegmatite melts require at least 98% fractionation of the initial G4-granite composition (Hulsbosch et al. 2014). The spatial distribution at Gatumba, however, is somewhat haphazard and appears to represent telescoping rather than a regional zonation. The source is probably a buried granite that oucrops only in the southernmost part of the pegmatite swarm (Figure 2.42).
In central Africa, small-scale mining is limited to near-surface zones of supergene alteration with soft-rock ore. Unaltered hard-rock ore is as yet rarely exploited.
Tantalum-niobium placers are commonly derived from pegmatites and occur in their immediate surroundings. The main resource is usually cassiterite with tantalum and niobium as by-products. Tantalum of tin placers in Malaysia and Thailand takes the form of �strueverite� (tantalian rutile) [(Ti,Ta,Nb,Fe2+)O2] inclusions in cassiterite. This tantalum is recovered from slags of tin smelters.
Very high tantalum metal prices up to 450 US$/kg in the period 1999-2000 incited a wave of tantalum exploration that resulted in the discovery of many significant prospects. At the same time, previously known tantalum granites were explored in several countries. In the future, a number of new hard-rock mines may be producing from these sources. Exploration for deposits of niobium and tantalum is based on alluvial heavy mineral and geochemical surveys of rocks, soil and sediments (Sweetapple 2000). Ballouard et al. (2016) report that a Nb/Ta ratio of <5 appears to be a good marker to discriminate mineralized from barren peraluminous granites. Useful indicator minerals include white mica and tourmaline; the conspicuously blue, green and yellow Li-tourmaline elbaite can be a visual guide to highly differentiated, and therefore possibly mineralised pegmatites. Testing trace-element concentrations (mainly Li, Al, Ti, Ge, B) by LA-ICP-MS in quartz from inner zones seems to be the most efficient method of discriminating potentially fertile from barren pegmatites (Beurlen et al. 2014). Because of large variations and random distribution within single pegmatites, columbite chemistry appears to be of little use for tantalum prospecting (Beurlen et al. 2008). Hidden cupolas of tin granites can by found by geophysical methods at greenfield scales. Detailed gravimetric surveys at brownfields reveal exploration targets by subsurface modelling of roots, steep flanks or cupolas of granites, that may control magmatic-hydrothermal ore bodies (Chicharro et al. 2014).
Carbonatites, alkali complexes and layered mafic intrusions are located by a combination of remote sensing, geological mapping, and aerial radiation, gravimetric and magnetic surveys.
The world�s niobium reserves are large, with >98% located in Brazil. World mine production of 56,000 t niobium contained in concentrate (2015) is dominated by Brazil (90%, mainly from Arax� mine), followed by Canada. After the effects of the financial and debt crisis in 2008 the worlds� primary tantalum production in 2015 was still low at ~1200 t tantalum contained in concentrate (Bleiwas et al. 2015). Rwanda (50%), DR Congo (16%) and Brazil (12%) were the largest producers. Both the US and the EU are taking measures to better control conflict minerals, especially from the DR Congo and neighbouring states. Fingerprinting traded concentrates (Melcher et al. 2008) and other provisions such as certifying and tagging production from conflict-free sources are expected to inhibit illegal trade. The role of China as a producer is negligible but its role as a buyer is substantial (Bleiwas et al. 2015). Tantalum reserves* in the ground (>100,000 t) are largest in Australia, followed by Brazil (USGS 2016).
*NOTE: USGS �Reserves� are defined as that part of the reserve base, which could be economically extracted or produced at the time of determination. This is not identical to JORC and similar definitions.
Recent useful papers cited (other references are available on request)
Ballouard, Ch., Poujol, M., Boulvais, Ph. et al. (2016) Nb-Ta fractionation in peraluminous granites: A marker of the magmatic-hydrothermal transition. Geology 44, 231-234. http://geology.gsapubs.org/cgi/content/abstract/G37475.1v1?papetoc
Beurlen, H., Thomas, R., Rodrigues da Silva, M.R. et al. (2014) Perspectives for Li- and Ta-mineralization in the Borborema Pegmatite Province, NE-Brazil: A review. J. South American Earth Sci. 56, 110-127. DOI: 10.1016/j.jsames.2014.08.00
Bleiwas, D.I., Papp, J.F. & Yager, Th.R. (2015) Shift in global tantalum mine production 2000-2014. USGS Fact Sheet 2015 � 3079, 6 pp, http://dx.doi.org/10.3133/fs20153079.
Chicharro, E., Martın-Crespo, T., Gomez-Ortiz, D. et al. (2014) Geology and gravity modeling of the Logrosan Sn- (W) ore deposits (Central Iberian Zone, Spain). Ore Geology Rev. 65, 297-304. doi: 10.1016/j.oregeorev.2014.10.005
Dewaele, St. � N. Hulsbosch � Y. Cryns � A. Boyce � R. Burgess � Ph. Muchez (2015) Geological setting and timing of the world-class Sn, Nb-Ta and Li mineralization of Manono-Kitotolo (Katanga, Democratic Republic of Congo). Ore Geology Reviews 07/2015; 72. DOI:10.1016/j.oregeorev.2015.07.004
Fritz, H., Abdelsalam, M., Ali, K.A., et al. (2013) Orogen styles in the East African Orogen: A review of the Neoproterozoic to Cambrian tectonic evolution. J. African Earth Sciences 86, 65�106.
Hulsbosch, N., Hertogen, J., Dewaele, St. et al. (2014) Alkali metal and rare earth element evolution of rock-forming minerals from the Gatumba area pegmatites (Rwanda): Quantitative assessment of crystal-melt fractionation in the regional zonation of pegmatite groups. Geochim. Cosmochim. Acta 132, 349-347.
Lehmann, B., Halder, St., Ruzindana Munana, J., Ngizimana, J. de la Paix, Biryabarema, M. (2014) The geochemical signature of rare-metal pegmatites in Central Africa: Magmatic rocks in the Gatumba tin�tantalum mining district, Rwanda. J. Geochem. Exploration (Special Volume on Mining and the Environment in Africa) 144, 528-538.
Melcher, F., Graupner, T., G�bler, H.-E., et al. (2016) Mineralogical and chemical evolution of tantalum�(niobium�tin) mineralisation in pegmatites and granites. Part 2: Worldwide examples excluding Africa and an overview of global metallogenetic patterns. Ore Geology Rev. http://dx.doi.org/10.1016/j.oregeorev.2016.03.014Melcher F., Graupner T., G�bler H.-E., Sitnikova M.A., Henjes-Kunst F., Oberth�r Th., Gerdes A. & Dewaele S. (2015) Tantalum�(niobium�tin) mineralisation in African pegmatites and rare metal granites: Constraints from Ta�Nb oxide mineralogy, geochemistry and U�Pb geochronology. Ore Geology Rev. 64, 667-719. http://dx.doi.org/10.1016/j.oregeorev.2013.09.003
Nieder, R., Weber, T.K.D., Paulmann, I., Muwanga, A., Owor, M., Naramabuye, F.-X., Gakwerere, F., Biryabarema, M., Biester, H. & Pohl, W.L. (2014) The geochemical signature of rare-metal pegmatites in the Central Africa Region: Soils, plants, water and stream sediments in the Gatumba tin-tantalum mining district, Rwanda. J. Geochem. Exploration (Special Volume on Mining and the Environment in Africa) 144, 539-551. doi: 10.1016/j.gexplo.2014.01.025
Pohl, W.L., Biryabarema, M. & Lehmann, B. (2013) Early Neoproterozoic rare metal (Sn, Ta, W) and gold metallogeny of the Central Africa Region: a review. Applied Earth Science 122, 66-82. http://www.tandfonline.com/doi/full/10.1179/1743275813Y.0000000033
Stepanov, A.S., Meffre, S., Mavrogenes, J. & Steadman, J. (2016) Nb-Ta fractionation in peraluminous granites: A marker of the magmatic-hydrothermal transition: COMMENT. Geology 44, 1 July 2016; No. 7 e394 Open Access! http://geology.gsapubs.org/cgi/reprint/44/7/e394?etoc
Some Links that may be of Interest to You
Tantalum-Niobium International Study Center
Company working Greenbushes and Wodgina pegmatite mines (Australia)
Company working Mibra tantalum pegmatite mine in Brazil
Company working Araxa niobium mine (Brazil)
Chart of LME Tantalite Price 2007-2012
Minimum 25% Ta2O5, 0.5% maximum ThO2+U3O8
back to top
back to Contents
Home
